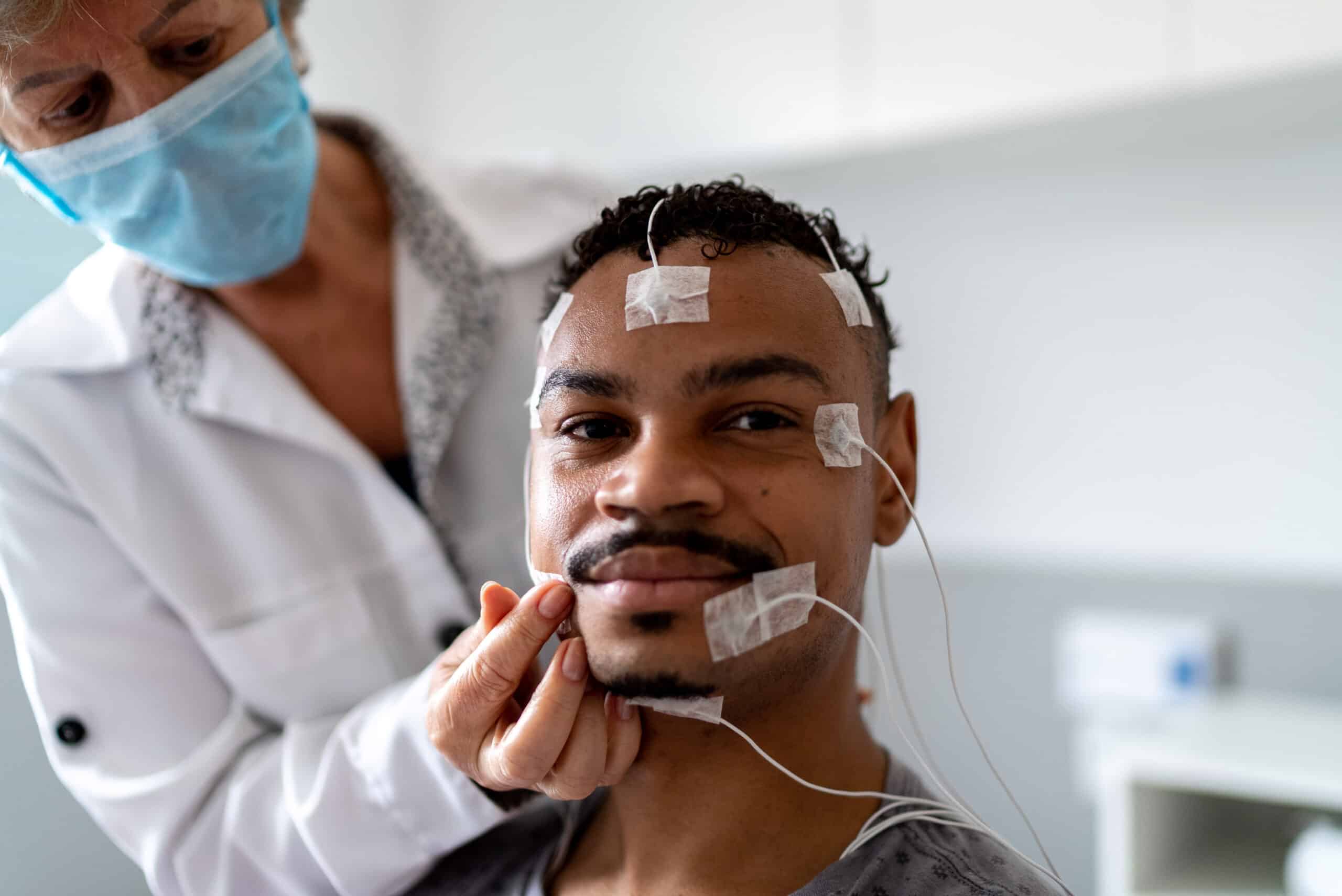
Imagine a world without insulin, chemotherapy, or COVID-19 vaccines. Without healthcare research—specifically clinical trials—many of today’s treatments would not exist.
So, what is a clinical trial? A clinical trial is a medical research study where researchers investigate the effectiveness of new medical approaches in real people, ranging from people with cancer to healthy volunteers. These trials help determine whether a treatment for a specific disease is safe and effective.
Clinical trials are the foundation of nearly every modern medical advancement. They not only lead to discoveries but also enhance current treatments, providing patients with better outcomes and increased hope.
In this article, we’ll break down what a clinical trial is, why it matters, and how you can get involved in life-changing research.
The Purpose of Clinical Trials
When it comes to the purpose of clinical trials, there are four main goals researchers keep in mind throughout the process:
- Evaluating medical treatments for safety and effectiveness, including drugs, devices, diagnostics, and preventative measures.
- Testing new therapies to determine how they compare to existing treatments.
- Determining the optimal dosage and method of administration for new or existing treatment options.
- Identifying potential side effects and monitoring risks associated with medical interventions.
“At a deeper level, clinical trials aim to close gaps in medical knowledge—especially in underrepresented communities—and to personalize medicine by understanding how variables like genetics, qualty of life, and social determinants affect health outcomes,” says Danielle Mitchell, CEO and Founder of Black Women in Clinical Research (BWICR).
The Four Phases of Clinical Trials
There are four trial phases, each with varying objectives. Here’s a breakdown of each phase:
- Phase 1: In this first stage, a new medical intervention is introduced to a small group of participants (usually 20 to 80 people). The primary goal of phase 1 clinical trials is to assess how the body responds and to identify any early safety concerns or side effects.
- Phase 2: Phase 2 clinical trials involve people of a larger sample size (approximately 100 to 300 individuals) to assess the effectiveness of the treatment. Researchers continue to monitor for adverse reactions and refine their understanding of the treatment’s safety.
- Phase 3: At this point in the clinical trial, the treatment is administered to a larger population (ranging from 1,000 to 3,000 participants). The goal of phase 3 clinical trials is to confirm effectiveness, track side effects in more detail, compare results with the standard of care, and gather the data needed for regulatory approval.
- Phase 4: After receiving FDA approval, the treatment becomes available to the public during phase 4 clinical trials. Researchers continue to monitor the medical intervention for long-term effects, rare side effects, and gather additional insights into how the treatment performs in everyday clinical use.
Who Participates in Clinical Trials?
Often, those who volunteer to participate in clinical trials have tried several treatments for their medical conditions and are seeking more effective care. “A lot of people who participate in clinical trials are at their last resort, and are willing to do a phase 1 trial at least,” says Ricki Fairley, Co-founder and CEO of Touch, The Black Breast Cancer Alliance.
Before joining a clinical trial, potential participants are selected based on inclusion and exclusion criteria. Inclusion criteria are the key features or characteristics that a person must meet to be eligible for a clinical trial.
Some examples of inclusion criteria include:
- Age
- Gender
- Race
- Ethnicity
- Location
- Lifestyle habits
Exclusion criteria are any characteristics or conditions that make a potential clinical trial participant ineligible. Certain medical conditions, medications, prior treatments, refusal, or inability to comply (e.g., transportation issues, mental impairment) can exclude someone from a clinical trial.
If a person is eligible to participate, the next step is for them to sign an informed consent form. “It provides detailed information about the trial’s purpose, risks, benefits, procedures, and their rights,” says LaToya Hinton-Howery, MPH, CEO and Research Director of NEXT Innovative Clinical Research. “It protects the rights, safety, and autonomy of participants. It’s a process of education and agreement that safeguards the well-being and dignity of every clinical trial participant.”
Informed consent in clinical trials is just one aspect of ethical considerations in clinical trial recruitment. Researchers and an ethics committee must also ensure participants’ privacy, avoid coercion, select participants fairly, and show respect for them.
The Importance of Clinical Trials
The most significant benefits of clinical trials are their contribution to medical breakthroughs. “They are vital to healthcare because every advancement—from a cancer-fighting drug to a COVID-19 vaccine—relied on clinical trials to determine if it truly works and for whom,” Mitchell says. “Without them, modern medicine would be based on theory rather than data.”
However, the impact of clinical trials relies on a diverse range of participants. Unfortunately, there’s still a lack of diversity in research, which disadvantages marginalized groups like the Black community. “When I looked at clinical trial records for the drugs that we take for breast cancer, a lot of them were made in the 70s and 80s, and there were no Black women in those trials,” Fairley says.
Mitchell asserts that diversity is essential in scientific research. “Without diverse participation, we risk developing treatments that do not work for everyone. Race, ethnicity, gender, age, and socioeconomic status all influence how diseases present and how treatments perform.”

Ethical Considerations in Clinical Trials
The ethics of clinical trials are guided by key principles, including respect for persons, beneficence (the principle of doing good to others), and justice. To ensure these standards are upheld to protect clinical participants, all clinical research must receive approval from the Institutional Review Board (IRB). IRB approval protects patients’ rights in clinical trials, including informed consent and ongoing safety oversight, which are essential to the ethical conduct of research.
Finding and Participating in Clinical Trials
Finding clinical trials can start with online databases like ClinicalTrials.gov, the National Cancer Institute (NCI), Karmanos Cancer Institute, and BlackDoctor.org’s Clinical Trial Resource Center for Black Americans.
If you’re wondering how to join a clinical trial, the process typically involves reviewing the study details, speaking with a research coordinator, and completing a screening process. “This process consists of pre-screening questions that ask basic and medical history questions to see if the individual meets general eligibility,” Hinton says.
When exploring clinical trials, it’s essential to evaluate the study’s credibility, understand the potential risks, and ask questions to make informed decisions.
Fairley also recommends getting a navigator. “A navigator is usually a nurse or social worker who helps you navigate your care,” she explains. “We use navigators to help recruit people in clinical trials by helping explain what’s happening, the drug involved, the science, and the informed consent to them and their families.”
Potential Benefits and Risks of Participating
There are several clinical trial pros and cons to consider before participating to ensure this experience is right for you.
Below are the potential benefits of joining a clinical trial, according to Hinton:
- Early access to new treatments: By joining a clinical trial, participants receive access to promising new treatments before they’re available to the public.
- High-quality, personalized care: Clinical trials often include expert oversight, regular health assessments, and comprehensive care, provided at no cost.
- Contributing to future breakthroughs: Participation plays a crucial role in advancing medical science, helping to shape better treatment for future patients.
- Gaining insight and confidence: Many participants find that they become more informed about their condition, which can lead to more empowered healthcare decisions.
In addition to the benefits, there are also several risks of clinical trials, including:
- Side effects from medical interventions may be unknown to researchers.
- Medical treatment may be ineffective or less effective than the current standard treatment.
- Even if a new treatment has benefits, it may not work for your medical condition.
Take time to weigh the possible benefits and risks of joining a clinical trial so you can make a well-informed decision that aligns with your personal health goals and lifestyle.
Frequently Asked Questions (FAQs)
Here are some common questions about clinical trials:
What is the difference between a clinical trial and other types of research?
Clinical trials test treatments in people. Other research may study data, animals, cells, or behavior without testing new medical interventions.
Is it safe to participate in a clinical trial?
Yes. Trials are carefully monitored and reviewed for safety, but, like any treatment, there are risks associated with it. You’ll be fully informed before joining.
Will I receive treatment if I participate in a clinical trial?
It depends. You may receive the new treatment, a standard option, or sometimes a placebo, depending on the study design.
Can I leave a clinical trial at any time?
You can quit a clinical trial at any time, no questions asked.
Who pays for clinical trials?
Government agencies, hospitals, or drug companies fund most clinical trials. Participants typically don’t pay for the treatment or related care.
Final Thoughts
Clinical trials are research studies involving human participants that are crucial to the development of new treatments and the improvement of care. They help advance medical knowledge and provide participants with an opportunity to contribute to future breakthroughs. To learn more, consider connecting with a patient advocacy group related to your medical condition—such as Touch, The Black Breast Cancer Alliance—or talk to your doctor. If you’re ready to take the next step, visit BlackDoctor.org’s Clinical Trial Resource Center explore studies near you.