This isn’t your average app. This app heals… Clinically proven to increase abstinence in patients suffering from substance abuse and expand outpatient retention in treatment programs, this app is a therapeutic digital wonder for substance use disorders.
The U.S. Food and Drug Administration has approved its first mobile app to help treat substance abuse, the agency stated in a news release. Approval of the app was given to Pear Therapeutics, based in Boston and San Francisco.
The reSET application is designed to help treat abuse of alcohol, cocaine, marijuana and stimulant medications. But the app is not intended for opioid dependence, the FDA said.
Citing the Substance Abuse and Mental Health Services Administration, the FDA said criteria for Substance Use Disorders (SUD) are met whenchronic use of these substances causes "significant impairment, such as health problems, disability, and failure to meet major responsibilities at work, school or home."
The newly-approved app delivers behavioral therapy that's designed to "increase abstinence from substance abuse and increase [participation] in outpatient therapy programs," the FDA said.
"This is an example of how innovative digital technologies can help provide patients access to additional tools during their treatment," said Carlos Peña, Ph.D., director of the FDA's Division of Neurological and Physical Medicine Devices.
The agency said it reviewed a 12-week clinical study involving nearly 400 people. Among those who used the app, 40.3 percent abstained from further alcohol, cocaine, marijuana or stimulant use, compared with 17.6 percent among those who did not use the app.
How does it work?
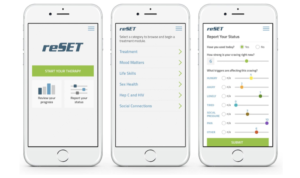
reSET is a prescription digital therapeutic designed toprovide neurobehavioral therapy based on the Community Reinforcement Approach (CRA) for patients with SUD. CRA is a specific form of cognitive behavioral therapy designed for patients with SUD.
reSET combines CRA for therapy and fluency learning to reinforce concept skills that can be implemented in conjunction with Contingency Management. This duo combination helps to support and push patients with SUD into achieving abstinence and retention in outpatient SUD treatment.
Patients are able to download the app on their cell phone, activate the product with a prescription access code provided by their physician or medical provider, thenproceed to a 12-week therapy. The patient’s physicians or medical providers can monitor the patient’s treatment and progress from a clinic dashboard as they progress through various modules and responses to questions.
In 2016, approximately 20.1 million people aged 12 or older had a substance use disorder related to their use of alcohol or illicit drugs in the past year. Abuse of and addiction to alcohol, nicotine, and illicit and prescription drugs cost Americans more than $700 billion a year in increased health care costs, crime and lost productivity, and contribute to the death of more than 90,000 Americans.
According to the Substance Abuse and Mental Health Services Administration (SAMHSA), only about 1 in 10 people who needed substance use disorder treatment received care at a specialty facility in the past year (10.6 percent).
If you feel you may have a substance use disorder, seek help now at SAMHSA.gov.
SOURCE:
SAMHSA.gov, Substance Abuse and Mental Health Services Administration
FDA.gov, press release, U.S. Food and Drug Administration, Sept. 14, 2017
Peartherapeutcs.com, Pear Obtains FDA Clearance of The First Prescription Digital Therapeutic to Treat Disease, Sept. 14, 2017