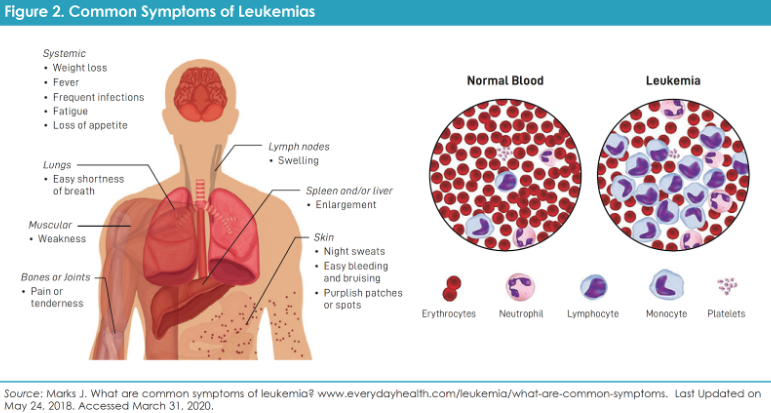
Chronic myeloid leukemia (CML) is a blood cancer that starts in the bone marrow when a genetic change creates an overactive protein called BCR-ABL1. This “Philadelphia chromosome” fusion protein drives CML cells to grow out of control. The good news is that CML has been transformed by targeted drugs. Before 2001, CML was often deadly – today, thanks to medicines taken by mouth, it can usually be managed like a chronic condition. About 9,000 people are diagnosed each year in the U.S., and over 50,000 are living with CML in remission. As the Leukemia & Lymphoma Society notes, new CML drugs have turned the disease “from life-threatening…to a chronic condition that can be managed with lifelong oral therapy”.
How Targeted Pills Changed the Game
The cornerstone of CML treatment is tyrosine kinase inhibitors (TKIs), a class of pills that block the BCR-ABL1 protein. Imatinib (Gleevec®) was the first TKI approved in 2001, and it dramatically improved outcomes. Since then, second-generation TKIs like dasatinib, nilotinib, and bosutinib have joined the lineup. These drugs work by binding to the ATP site of the BCR-ABL1 enzyme, shutting it down. Clinical studies show that imatinib and the newer 2nd-generation TKIs greatly improved patients’ survival. Ten-year progression-free survival is now often in the mid-80 percent range, meaning most people live long lives with the disease under control. Compared to imatinib, the 2nd-generation TKIs usually produce a faster and deeper response, which opens the door to stopping treatment for some patients. Importantly, TKIs have made it possible for many people to live well with CML on a daily pill, as long as side effects are managed and blood tests show the disease is responding.
RELATED: 7 Little-Known Side Effects of CML Treatment – & How to Handle Them
The 2025 Breakthrough: New TKIs with Fewer Side Effects
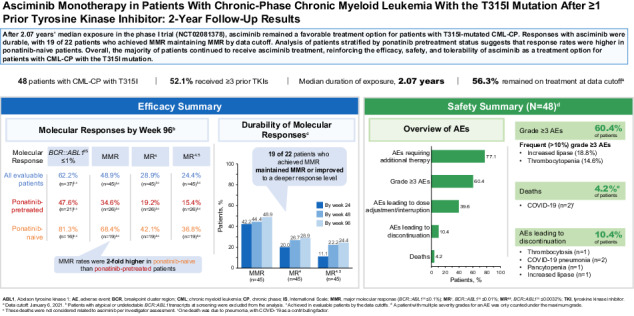
Most frontline CML care still starts with a TKI pill, but the newest TKI – asciminib (Scemblix®) – is changing the conversation. Asciminib works differently: it targets the “myristoyl pocket” of BCR-ABL1 (a unique spot on the protein) rather than the usual ATP binding site. In late 2024, the FDA approved asciminib for newly diagnosed chronic-phase CML. This is big news for 2025. In a large trial (ASC4FIRST), asciminib produced higher rates of deep molecular response at one year than standard TKIs, and with better tolerability. Patients on asciminib were more likely to reach key milestones, and side effects like gut upset and fatigue were generally mild and often happened only early on.
Leukemia experts highlight that asciminib’s superior safety and efficacy helped win its new approval. The FDA and LLS report that asciminib showed “superior efficacy and safety” as initial therapy. In practice, this means less diarrhea, fluid retention, or blood pressure problems than some older TKIs, and less risk to the heart and vessels. For patients who have the rare T315I mutation (which makes most TKIs stop working), asciminib has also proven effective and is approved for those cases. In short, asciminib gives doctors a powerful new option that many patients can start taking instead of older drugs.
RELATED: 5 Ways to Make Your CML Treatment More Affordable
Treatment-Free Remission: A Realistic Goal
One of the most hopeful developments in CML is the ability for some patients to stop therapy altogether. This is called treatment-free remission (TFR). The idea is that if a patient has been on a TKI long enough to reach a deep molecular response (very low BCR-ABL1 levels), doctors may safely pause the drug under close monitoring. Early trials, started around 2007, showed that roughly half of the patients who tried stopping imatinib remained in remission without medication for years. For example, about 52 percent of people were still disease-free six months after stopping, and 50 percent at two years. These results were very encouraging and have held up with newer TKIs: in one study, 61% were still relapse-free at six months, and 50 percent at 24 months.
This means that many patients with a stable deep response can aspire to live without daily pills. Doctors are now carefully defining who can try TFR – usually those who have had an undetectable (or near-undetectable) cancer signal for at least two years. Success rates are higher for patients who started on a 2nd-generation TKI (like dasatinib or nilotinib) or asciminib because those tend to achieve deeper remissions. (One report showed asciminib led to more patients hitting molecular milestones than imatinib .) Researchers are studying how to safely stop newer drugs, too. Of course, if the cancer comes back (often seen by a tiny rise in BCR-ABL1 on a blood test), therapy can be restarted promptly. Importantly, experts stress that trying treatment-free remission should be a shared decision and done under a doctor’s guidance – it’s not “going off meds forever” without a plan.
RELATED: What to Expect After CML Treatment: A Guide to Post-Treatment Life
Stories of Hope and Strength
Living with CML can be an emotional journey, and hearing from others can bring comfort and inspiration. Mel Mann’s story is one such example. Diagnosed in 1995 and told he had just three years to live, he feared for his young family. As a Black American, he knew a bone marrow transplant was unlikely – “being an African American, my chances were slim,” he recalls, because there were so few Black donors. Mel entered a clinical trial in 1998 and received treatment that wasn’t yet standard. Almost 28 years later, he is thriving, and he credits that trial with saving his life. Today, Mel urges others to consider trials: “Clinical trials allow you to get tomorrow’s medicine today,” he says. His journey underscores the power of new therapies and of advocating for yourself.
Many Black patients find extra motivation in community figures. NBA legend Kareem Abdul-Jabbar, who was diagnosed with CML in 2008, emphasizes staying proactive. He says, “A strong support network and managing stress are essential to living well with the disease.” Kareem’s story reminds us that, alongside new drugs, a healthy lifestyle and good care coordination matter too.
Historically Black patients have indeed faced tougher odds – studies show five-year CML survival for Black Americans is around 73 percent, compared to about 84 percent in other groups. These gaps reflect barriers in care, not biology. The bright side is that with today’s treatments, everyone has reason to hope. The newest therapies and clinical trials are available regardless of race, and spreading awareness in our community can help close those gaps.
RELATED: Lifestyle Changes After Chronic Myeloid Leukemia (CML) Treatment
Talking with Your Doctor: Empower Yourself
You don’t have to navigate CML alone. Ask questions and be involved in decisions. For example, say, “I’ve heard about a new drug called asciminib – could that be right for me?” Or if you’ve had any side effects or concerns, “Are there different TKIs or dose adjustments that might help my situation?” Your doctor can explain the latest options and may have clinical trials you haven’t considered.
Remember, reaching for TFR isn’t taboo – you can discuss whether you’re a candidate for stopping medication after a deep response. And if you feel symptoms like fatigue or anxiety, don’t hesitate to bring them up. Emotional support is part of care, too.
Finally, consider getting a second opinion at a CML specialty center or talking to an information specialist at organizations like the Leukemia & Lymphoma Society. These experts keep up with the newest research.
CML patients today have more reasons than ever to be hopeful. Advances like asciminib and ongoing TFR research mean lives beyond daily pills are possible. Take the next step by asking your doctor about these options. And if you feel ready, ask about clinical trials – they can open doors to cutting-edge treatments that could change your life, just as they did for Mel Mann.
RELATED: Chronic Myeloid Leukemia: Symptoms, Causes, Diagnosis, and Treatment
Questions to Ask Your Care Team
- New options: “Are there newer TKIs or trials I should know about (for example, asciminib)?”
- Side-effect management: “Which side effects should I expect, and how can we manage them?”
- Treatment-Free Remission: “If my molecular response is deep, am I a candidate to safely stop therapy?”
- Support resources: “Can you refer me to a CML specialist or patient support group?”
- Monitoring plan: “How often will I need blood tests, and what milestones should we watch for?”
Further Resources
- Leukemia & Lymphoma Society (LLS): Free CML guides and information specialists (call 1-800-955-4572 or visit lls.org).
- American Society of Hematology (ASH): Patient resources on leukemia (hematology.org).
- ClinicalTrials.gov: Search for CML trials by location and criteria.
- CML Foundation (CML-foundation.org): Updates on research and patient support.
- BlackDoctor.org: Articles and videos on CML and clinical trials in the Black community.
By staying informed and speaking up, you can help ensure that you and your family benefit from the very best care. Ask your doctor about the latest CML treatments today.