
The Mayo Clinic is treating patients with COVID-19 with monoclonal antibody treatments. Monoclonal relates an antibody derived from a single cell in large quantities for use against a specific antigen.
An antigen is a toxin or other foreign substance that induces an immune response in the body, especially the production of antibodies.
The Food and Drug Administration (FDA) issued an emergency use authorization to use bamlanivimab and casirivimab-imdevimab to treat confirmed COVID-19 in patients who have mild or moderate symptoms, and at a high risk of disease progression and hospitalization.
The treatments are given in the outpatient setting at infusion centers across Mayo Clinic's locations in the Midwest.
Dr. Raymund Razonable, a Mayo Clinic infectious diseases expert, discusses how the treatment works, who is eligible to receive it, and why testing for early detection of COVID-19 is so important. https://youtu.be/mFKwaQuFEZM
The monoclonal antibody treatments help keep patients out of Mayo Clinic hospitals and decrease the severity of the disease. To date, Mayo Clinic has infused over 400 patients.
"The FDA has given emergency use authorization only for high-risk individuals.
Those individuals are 65 years and older; have underlying conditions, such as diabetes or chronic heart disease, as well as those who have a compromised immune system, such as, patients with cancer and those who have undergone transplantation; those who are receiving high doses of steroids or other immunosuppressive drugs," says Dr. Razonable.
Patients receive one dose of this treatment through outpatient IV infusion.
Treatments last close to 2½ hours. Dr. Razonable says it's important for people to understand that the treatment is not metabolized in the kidneys or liver, and is not expected to interfere with other patient medications.
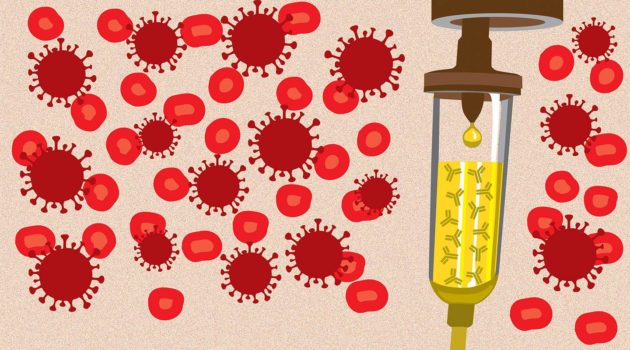
"Monoclonal antibodies are basically antibodies that will attach to the spike protein of the SARS-CoV-2 virus (the virus that causes COVID-19). Once it attaches to that spike protein, the virus cannot attach itself to the cells, thereby limiting the spread of the infection and preventing it from progressing further," explains Dr. Razonable.
"To be effective, this drug has to be given early, so that the protein of the virus can be bound to this antibody so that it prevents the infection from progressing," says Dr. Razonable.
There does come a point when it's too late to give the drug.
Dr. Razonable says the treatment no longer will be effective once patients require oxygen supplementation if they develop shortness of breath, or their oxygen saturation is low.
Testing to detect the infection and start treatment as early as possible in the course of the disease is important.
For more information and all your COVID-19 coverage, go to the Mayo Clinic News Network and mayoclinic.org.









