Sickle cell disease is one of the most common inherited blood disorders, affecting about 100,000 Americans, most of them black, and about 300 million people worldwide.
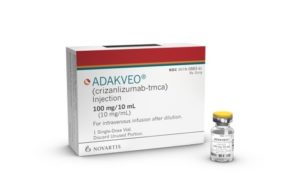
Just last month, the U.S. Food and Drug Administration (FDA) approved Novartis’ new treatment, Adakveo (crizanlizumab), to decrease the frequency of pain crises, or vaso-occlusive crises (VOCs), in adults and children age 16 years and older with sickle cell disease.
This approval, two months ahead of its scheduled decision date, has granted Novartis space to boast its impressive analysis for patient hospitalization frequency. Adakveo was able to win over the FDA’s approval with data generated in a study that shows the drug could reduce the number of VOC hospitalizations by 40%.
VOCs or pain crises are the periodic episodes during which red blood cells stick together, blocking blood from reaching organs and small blood vessels. This causes intense pain and cumulative organ damage that shortens the lives of people with the disease. “The duration and severity of these pain crises worsens with aging. Often patients die during one of these crises,” said Dr. Biree Andemariam, chief medical officer of the Sickle Cell Disease Association of America.
Ameet Mallik, the Novartis’ head of U.S. oncology and blood disorders stated that VOCs send U.S. patients to emergency departments about 200,000 times per year. About 85% are hospitalized for days to a week, running up big bills.
The study showed Adakveo improved the proportion of patients who weren’t hospitalized at all. About 50% of the patients taking the drug never went to the hospital compared to about 30% of participants who were on placebo. This information was presented by Novartis at the American Society of Hematology annual meeting this past Sunday.
“We wanted to dig further” into the “numerically interesting results” turned up in the company’s phase 2 Sustain Trial and“all the data cuts point in the same direction,” Andrew Cavey, who leads Novartis’ benign hematology development programs.“The next phase of work is we need to generate more data,” he added.
U.S. regulators green-lighted Adakveo in mid-November on the back of results showing the drug could slash the median annual rate of VOCs by 45% compared with placebo.
"We worked extremely hard to get the approval fast,” Cavey said of the go-ahead, which came a couple of months before the FDA’s decision deadline. And the pre-ASH timing was a “lucky” result.
There’s only, maybe with EHA, twice a year where the entire global community of hematologists is in one place,” he said.“The ability to disseminate the data, share the news—because this is a U.S. approval, to do so at ASH is that much more powerful.”
For more information on Adakveo and how it works, keep reading!
New Sickle Cell Drug, ADAKVEO, Approved!

The U.S. Food and Drug Administration (FDA) approved Novartis’ Adakveo (crizanlizumab) to decrease the frequency of pain crises, in adults and children age 16 years and older with sickle cell disease. Novartis indicates it expects to make the therapy available in the coming weeks.
Sickle cell disease is a genetic condition that affects the body’s red blood cells. It occurs when a child receives two sickle cell genes—one from each parent. In someone living with this disease, the red blood cells become hard and sticky and look like a C-shaped farm tool called a “sickle”. This can lead to acute pain episodes called VOCs, in addition to life-threatening complications. Those life-threatening conditions include stroke, vision loss, blood clots in the lungs, and infections.
It is estimated that:
- SCD affects approximately 100,000 Americans.
- SCD occurs among about 1 out of every 365 Black or African-American births.
- About 1 in 13 Black or African-American babies is born with sickle cell trait (SCT)
Adakveo is a monoclonal antibody that binds to P-selectin on the surface of the activated endothelium—the inside walls of blood vessels—and platelets. The approval was based on the results of the 52-week SUSTAIN trial. The trial showed that Adakveo lowered the median annual rate of VOCs to 1.63 compared to 2.98 for the placebo group, a reduction of 45%.
“The approval of Adakveo marks a new era in the treatment of sickle cell disease, a genetic condition that places an extraordinary burden of unpredictable pain crises on patients and their families,” said Susanne Schaffert, president of Novartis Oncology. “The stories we have heard from patients about their sickle cell pain crises are devastating. We are pleased to help reimagine medicine together with the sickle cell community and offer new hope for fewer VOCs.”
Sickle cell disease affects about 100,000 people in the U.S., according to the U.S. Centers for Disease Control and Prevention (CDC). A 2009 study published in the journal Pediatric Blood and Cancer noted that in 2005, sickle disease cost an average of $11,702 for children under Medicaid and $14,772 for patients with employer-sponsored insurance.
The projected cost of the drug is $2,357 per vial, which the expected dosage of three or four vials per month coming to $7,071 or $9,428 per month. It is prescribed based on weight. Payers are likely to be more than willing to pay it because, in addition to decreasing the pain crises, the drug decreased the median days a patient spent in the hospital each year by 42%.
ADAKVEO is used
- in people 16 years of age and older who have sickle cell disease
- to help reduce how often certain episodes (crises) happen
It is not known if ADAKVEO is safe and effective in children under 16 years of age.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about ADAKVEO?
ADAKVEO may cause serious side effects, including infusion reactions.
Infusion reactions. Infusion reactions may happen within 24 hours of receiving an infusion of ADAKVEO. Tell your health care provider right away if you get any of the following signs and symptoms of an infusion reaction:
- fever
- chills or shivering
- nausea
- vomiting
- tiredness
- dizziness
- sweating
- hives
- itching
- shortness of breath or wheezing
Your doctor or health care provider may monitor you for signs and symptoms of infusion reactions.
ADAKVEO may interfere with a blood test.
Tell your health care provider that you are receiving ADAKVEO before having any blood tests. ADAKVEO may interfere with a laboratory test to measure your platelet counts.
Before receiving ADAKVEO, tell your health care provider about all of your medical conditions, including if you are
pregnant or plan to become pregnant. It is not known if ADAKVEO may harm your unborn baby
are breastfeeding or plan to breastfeed. It is not known if ADAKVEO passes into your breast milk. You and your health care provider should decide the best way to feed your baby during treatment with ADAKVEO
Tell your health care provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
The most common side effects of ADAKVEO include:
- nausea
- back pain
- joint pain
- fever
These are not all of the possible side effects of ADAKVEO. For more information, ask your health care provider or pharmacist.
Call your doctor for medical advice about side effects.